Role of cytomegalovirus UL25 protein family members for cell-to-cell spread in persisiting infection
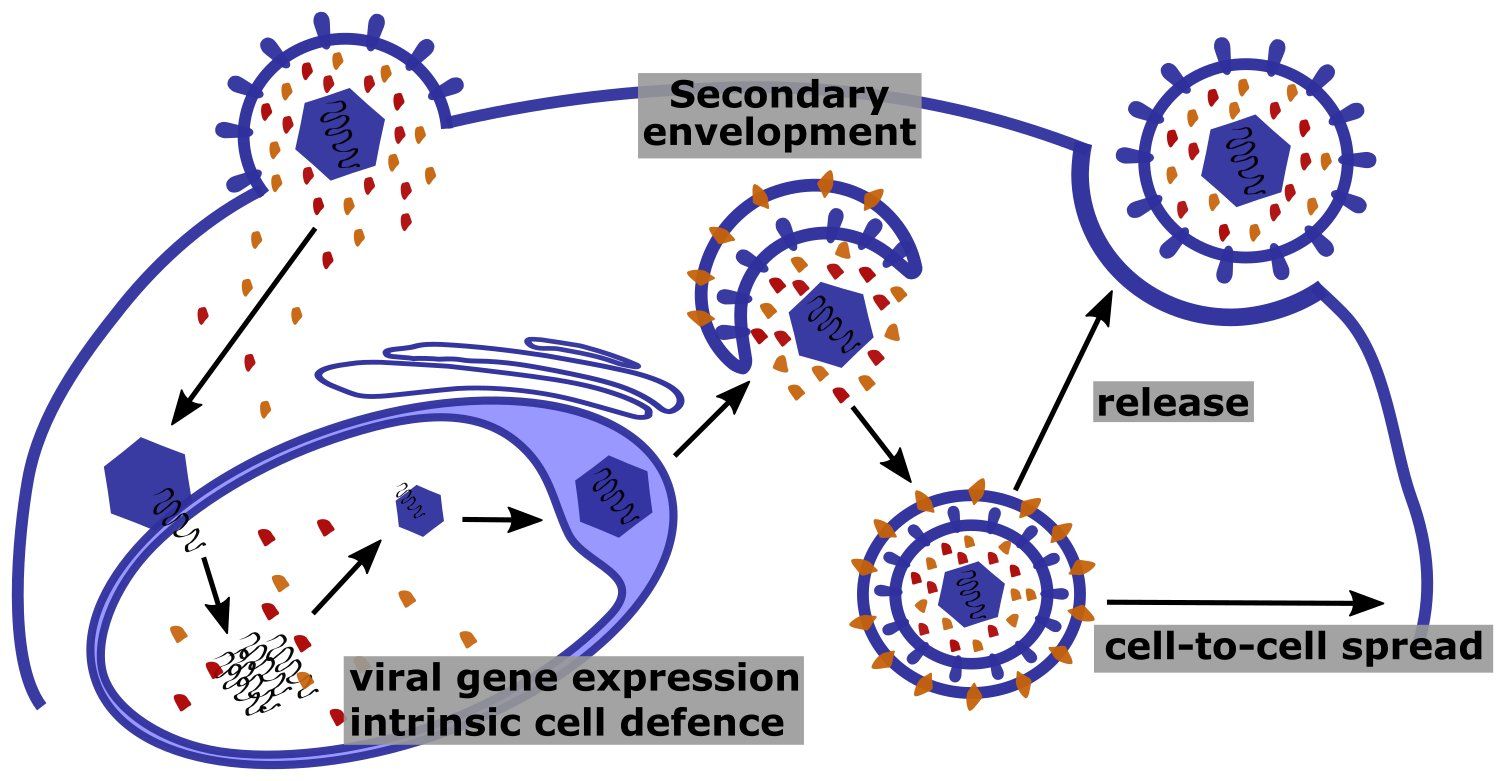
Functions of tegument proteins in the CMV infection cycle.
Tegument proteins such as the UL25 family members (labelled orange and red) are delivered into the cell upon viral entry. Within the cell nucleus tegument proteins can counteract intrinsic defense mechanisms and promote viral gene expression. Late in infection newly synthesized tegument proteins localize to cytoplasmic membranes to get incorporated into virions. Moreover, binding of tegument proteins to the cytoplasmic side of secretory vesicles allows them to interact with components of the cytoskeleton to facilitate egress of virions and spread to adjacent cells.
In industrialized countries cytomegalovirus (CMV) infects approximately half of the human population. However, due to the mild symptoms, most of the infected individuals are unaware of the infection. As a true member of the herpesvirus family, after initial infection, HCMV stays dormant in specific cell types. Occasionally, the virus wakes up (“reactivates”) and is shed in body fluids such as saliva to get transmitted to other individuals. In immunocompromised patients (e.g., transplant recipients) or following congenital infection of unborn children, CMV can lead to severe life threatening complications. Understanding how the virus persists, i.e. enters the dormant state (“latency”) and reactivates from it, is a prerequisite to develop new intervention strategies.
CMV infects a range of different cell types and is closely associated with these cells. Regardless of whether infection is initiated by newly entering virions or by reactivation from latency, the virus must reprogram the infected cells to ensure efficient transmission of viral progeny to neighboring cells. In tissues, CMV is mainly transmitted through direct cell-cell contacts. The way in which CMV mediates cell-to-cell transfer is not well understood, but it is believed that the virus utilizes the cytoskeleton of infected cells for this process.
By combining virological, biochemical and cell biological techniques we aim to understand how the cytomegalovirus UL25 proteins – a class of viral tegument proteins – alter the cytoskeleton and interfere with cellular pathways in order to facilitate viral cell-cell transfer and to ensure persistent infection.
Scientific approach
CMV infection leads to changes in the actin cytoskeleton, causing the typical enlargement and rounding of infected cells. This process is so characteristic that it was eponymous for the virus.
We observed that a murine CMV (MCMV) mutant lacking the M25 gene is unable to induce the typical cytopathic effect. Moreover, spread of the mutant in cell cultures is impaired, and the mutant is attenuated in vivo. The MCMV M25 protein, a member of the CMV UL25 protein family, is one of the most abundant tegument proteins of this virus. Immediately after viral entry, the M25 protein is transported to the nucleus, where it associates with subnuclear structures (ND10 bodies). Later in the infection cycle, the M25 protein is also detected in the cytoplasm of infected cells. Upon expression of the M25 protein independent of viral infection morphological changes of cells were induced, confirming that the M25 protein causes remodeling of the cytoskeleton and promotes cell-to-cell spread of MCMV.
To examine the mode of action of M25, co-immunoprecipitation experiments coupled to SILAC-based mass spectrometry were performed and potential cellular binding partners of M25 were identified. We will now analyze which of the protein-protein interactions of M25 cause the cytoskeletal changes and which cellular signaling pathways are affected. Moreover, we will determine if the identified M25 interaction partners and M25-regulated cellular pathways are conserved among UL25 protein family members of human CMV. Understanding the molecular basis of M25 interference with cellular signaling pathways will help us dissect the mechanisms underlying cell-to-cell spread of CMV and thus persistence of the virus.
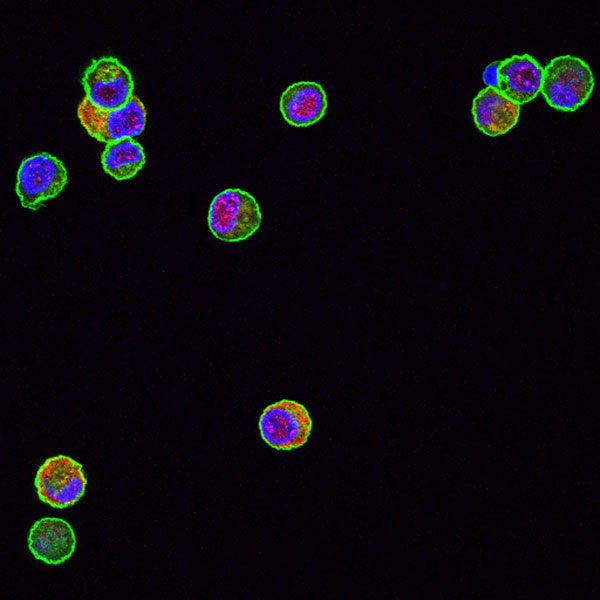
The MCMV M25 deletion mutant lacks the ability to induce the typical cytopathic effect. Fibroblast infected with wild-type MCMV.
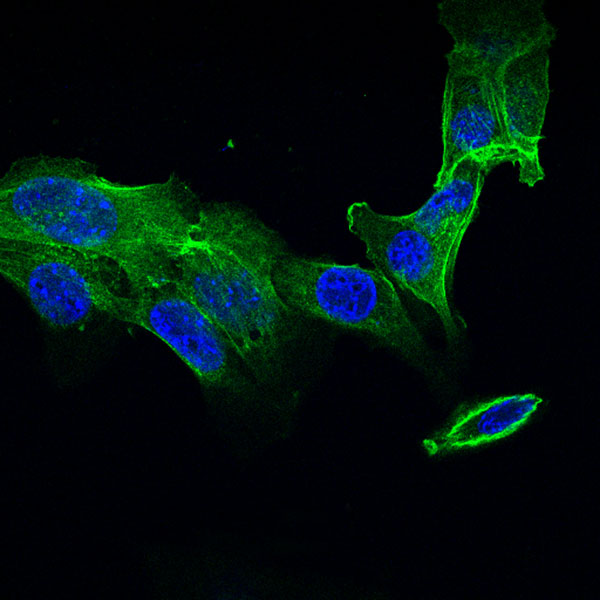
The MCMV M25 deletion mutant lacks the ability to induce the typical cytopathic effect.
Fibroblast cells were infected with wild-type MCMV (left) or the M25 deletion mutant (right) and labelled with an M25-specific antibody (red) and with phalloidin (green) to mark actin fibers.
Martin Messerle is talking about his research at CRC 900
Martin Messerle joined the CRC at the start of the second funding period in 2014 with project C6. In this project he and his group are investigating the roles of cytomegalovirus proteins in host defence.
Publications of the project C6
Contact

Prof. Martin Messerle
Institute of Virology
Hannover Medical School
Carl-Neuberg-Str. 1
30625 Hannover
+49 511 532-4320
Messerle.Martin@mh-hannover.de