The interplay of EBV-induced γδ and αβ T cells during chronic EBV reactivation, PTLD, and EBV-directed cellular therapy in transplant recipients
Epstein Barr Virus (EBV) can lead to infectious mononucleosis (“Pfeiffer’s glandular fever”). After a first infection usually in childhood to adolescence, the virus nests in the B cells of the immune system for life and is controlled by the healthy immune system. In case of a weakened immune system, e.g. after transplantation and in patients who need to take immunosupressive drugs, EBV can multiply uncontrollably and can finally lead to the development of malignant tumors (lymphoproliferative disorders).
T cells of the immune system can control EBV and the anti-EBV immune response has been well described for αβ T cells. However, there is increasing evidence that also γδ T cells can control EBV virus control. In this project, we investigate the contribution of γδ T cells to the fight against EBV infection in healthy patients and how this interaction with αβ T cells changes under immunosuppression. One therapeutic option for transplant patients with EBV-associated problems is the transfer of purified EBV-specific T cells from healthy donors. These are usually almost exclusively αβ T cells, in this study we aim at characterizing the role of the patient’s own γδ T cells in the efficacy of the transferred EBV-specific T cells in the patient.
Scientific work programme
For EBV-associated lympho proliferation, we can infect B cells with EBV in the laboratory and then test the immune response in different constellations. In addition to the sheer number of EBV-specific αβ and γδ T cells, their appearance is examined using surface markers and their function in cytokine and cell lysis tests. Sequencing of the T cell receptors will show which specific T cell clones are stimulated and expanded by EBV. Insights gained in this system will be transferred to a cohort of kidney and liver transplant patients who have either successfully overcome EBV infection or chronically detect EBV in their blood among the necessary immunosuppressive drugs.
In a final step, we will follow the progression of αβ and γδ T cells in patients receiving EBV-specific T cell transfer. We will evaluate to what extent γδ T cells, which are not transferred with the preparation, contribute to the success of cell therapy. Through T-cell receptor sequencing, we want to investigate whether there are specific sequences for EBV and compare them to sequences in other viral diseases (e.g., CMV, TP B8, hepatitis) to identify possible common sequences.
In the long term, we expect to gain insights into spurious regulation in immunocompromised patients and to improve the efficacy of T cell preparations by supporting additional cell fractions.
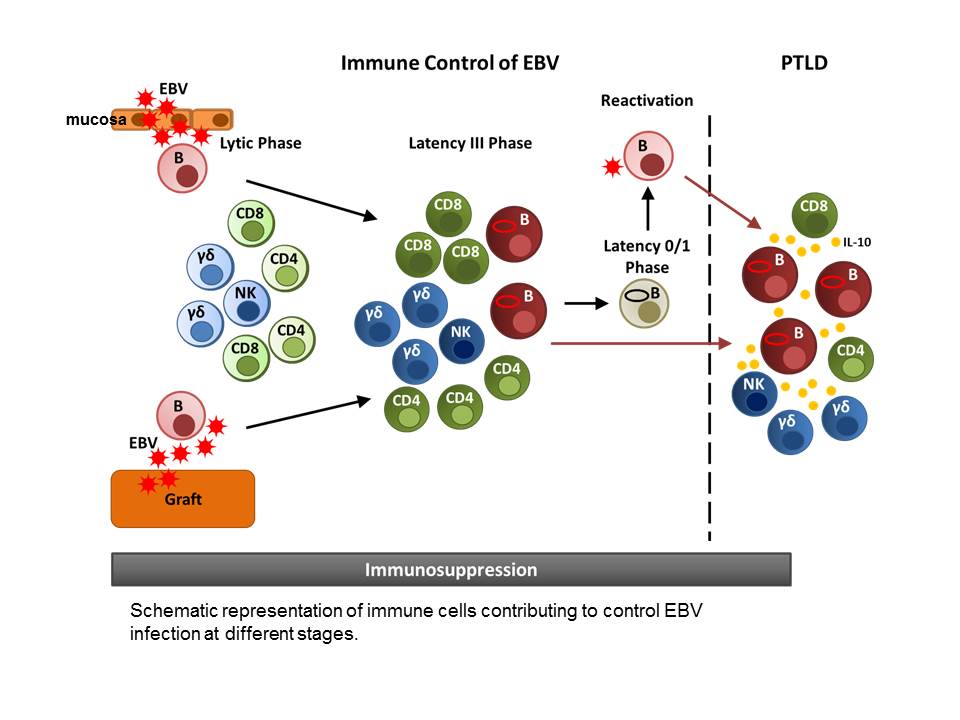
Schematic representation of immune cells contributing to control EBV infection at different stages.
Britta Maecker-Kolhoff is talking about her research at CRC 900
Britta Maecker-Kolhoff joined the CRC in the third funding period in 2018. She is jointly leading the project B11 with Britta Eiz-Vesper and Sarina Ravens, which is concerned with the interactions between certain types of T cells during chronic reactivation of the Epstein-Barr Virus (EBV) and EBV-directed cellular therapy in transplant patients.
Sarina Ravens is talking about her research at CRC 900
Sarina Ravens joined the CRC in the third funding period in 2018. She is jointly leading the project B11 with Britta Eiz-Vesper and Britta Maecker-Kolhoff, which is concerned with the interactions between certain types of T cells during chronic reactivation of the Epstein-Barr Virus (EBV) and EBV-directed cellular therapy in transplant patients.
Britta Eiz-Vesper is talking about her research at CRC 900
Britta Eiz-Vesper joined the CRC in the third funding period in 2018. She is jointly leading the project B11 with Britta Maecker-Kolhoff and Sarina Ravens, which is concerned with the interactions between certain types of T cells during chronic reactivation of the Epstein-Barr Virus (EBV) and EBV-directed cellular therapy in transplant patients.
Publications of the project B11
Contact

Prof. Britta Maecker-Kolhoff
Department of Pediatric Hematology and Oncology
Hannover Medical School
Carl-Neuberg-Str. 1
30625 Hannover
+49 511 532-6747
Maecker.Britta@mh-hannover.de

Dr. Sarina Ravens
Institute of Immunology
Hannover Medical School
Carl-Neuberg-Str. 1
30625 Hannover
+49 511 532-5240
Ravens.Sarina@mh-hannover.de

Prof. Britta Eiz-Vesper
Institute of Transfusion Medicine
Hannover Medical School
Carl-Neuberg-Str. 1
30625 Hannover
+49 511 532-9715
Eiz-Vesper.Britta@mh-hannover.de