Immune modulation and colonization of sensory ganglia by alphaherpesviruses
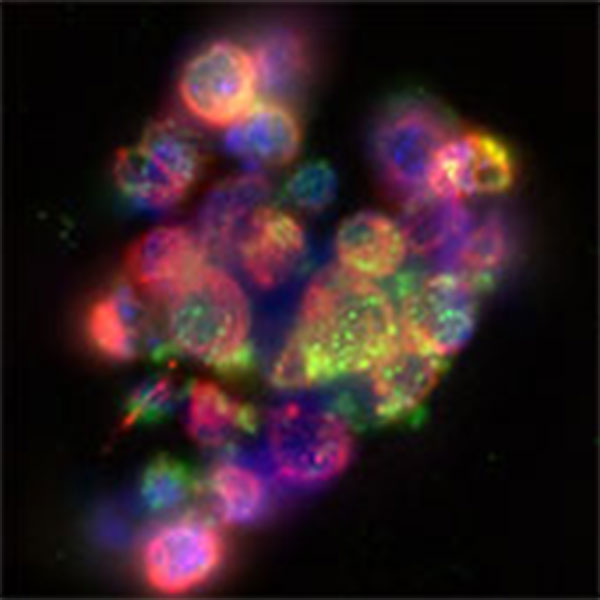
Figure 1: Vero cells infected with HSV-2 expressing mCherry. Magenta, tubulin; Blue, DAPI; Green, anti-HSV serum; Red, mCherry
Varicella zoster virus glycoprotein C increases chemokine-mediated leukocyte migration. Herpesviruses have co-evolved with their hosts during millions of years. Therefore, they are repositories of information that can be exploited in two ways. First, to understand viral processes such as viral replication cycle, immune modulation and pathogenesis to facilitate the development of novel antiviral strategies. Second, understanding the viral strategies of host interaction provides relevant knowledge about the functioning of the host, facilitating the discovery of host proteins that control basic processes of cellular function.
Among the human herpesviruses, herpes simplex virus type 1 and 2 (HSV-1 and HSV-2, respectively) and varicella zoster virus (VZV) colonize and persist within neurons, being able to modulate the host’s nervous system. The outcome of infection is highly dependent on the modulation of and interaction with the immune system and the successful colonization of the nervous system. After primary infection these viruses establish lifelong latency in specific subtypes of neurons and can reactivate by specific stimuli.
Most adults are infected with VZV and HSV-1, acquiring the viruses during childhood. HSV-2, however, is normally transmitted sexually and infects a lower number of individuals. HSV-1 and HSV-2 cause a variety of diseases, such as cold sores, herpes simplex keratitis leading to blindness, painful genital herpes or encephalitis. VZV causes varicella during primary infection and zoster, associated with acute pain, following reactivation. A percentage of zoster patients suffer from post herpetic neuralgia, chronic pain that last for months after the disappearance of the zoster rash.
The long-term goal of our present research is to understand how HSV-1, HSV-2 and VZV modulate the immune and nervous systems of the host in order to develop novel antiviral drugs, vaccines and immunomodulators. We focus on the modulation of chemokines and neurotrophic factors, proteins involved in the crosstalk between the immune and nervous systems.
Scientific work programme
Herpes simplex virus type 1 and 2 (HSV-1 and HSV-2, respectively) and varicella zoster virus (VZV) are highly prevalent human neurotropic viruses that establish latency in neurons of the peripheral nervous system. Colonization of the nervous system is required for pathogenesis and several reports indicate that HSV-1 and HSV-2 have a preference for establishing latency in distinct types of neurons. These viruses modulate the immune system during primary infection, establishment of latency and reactivation.
HSV-1 and HSV-2 glycoprotein G (gG1 and gG2, respectively) bind chemokines and enhance their activity, increasing leukocyte migration. Similarly, both bind nerve growth factor, but only gG2 enhances its activity, increasing the growth of neurites expressing its receptor, TrkA. This may facilitate the infection of particular subtypes of neurons and pathogenesis since TrkA is normally expressed in peptidergic neurons associated with the induction of pain, a symptom characteristic of genital herpes. To determine whether gG1 and gG2 could play a role in virus tropism and pathogenesis we generated viral mutants lacking gG and we swapped the gG locus between both viruses in collaboration with Beate Sodeik (Project C2). All these recombinant viruses express mCherry and Gaussia luciferase to facilitate their detection in vitro, ex vivo and in vivo. We characterized these recombinant viruses in vitro showing that lack of gG expression does not affect viral replication.
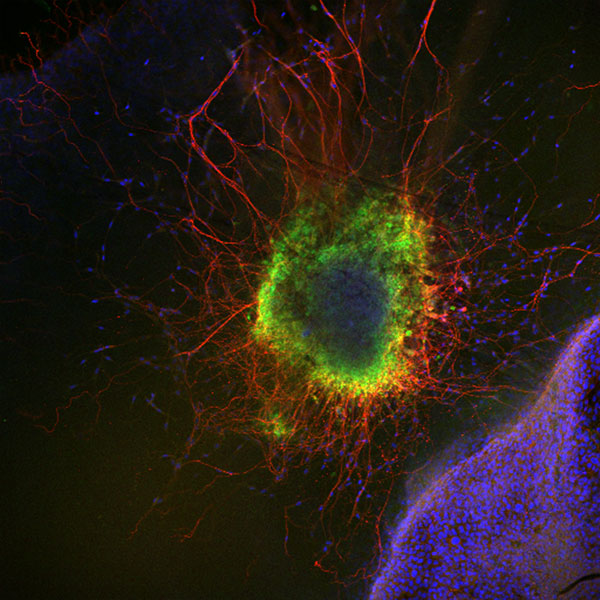
Figure 2: Mouse superior cervical ganglion in the proximity of the keratinocyte cell line HaCat. The expression of TrkA, the NGF receptor, was detected. Green, TrkA; Red, beta-III-Tubulin; Blue, Dapi.
We are currently determining the effect of these modifications on the infection of specific subsets of mouse neurons ex vivo, using ganglion explants and microfluidic devices. We are investigating the role of gG on pathogenesis, focusing on immune modulation and the colonization of the nervous system in vivo, in collaboration with Antonio Alcami (CBM, Madrid, Spain). Our results will determine the relevance of chemokine and neurotrophic factor modulation by HSV and will provide information to develop novel antiviral strategies.
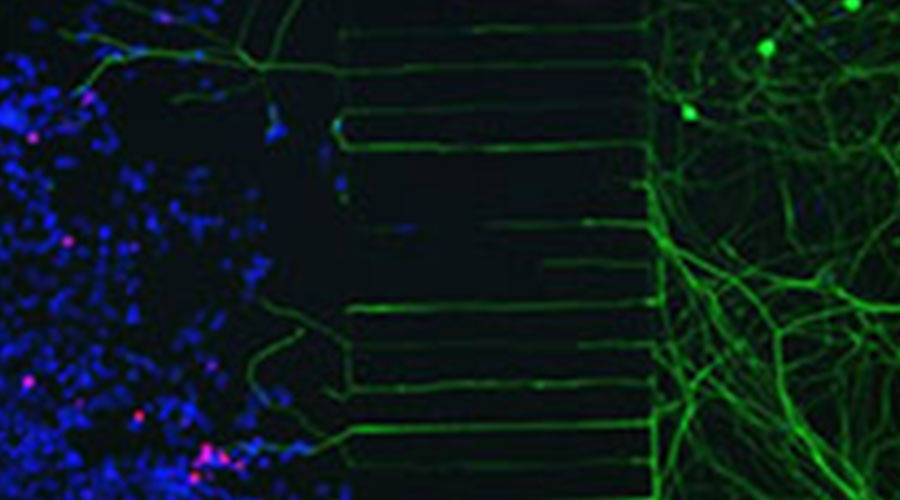
Figure 3: Microfluidics device showing 293-T cells infected with HSV-2 expressing mCherry on the left side of the microgrooves and neuronal bodies on the right side. Nerve endings go through the microgrooves and reach the infected cells. Red, mCherry; Green, beta-III-Tubulin; Blue, Dapi.
Other collaborations within CRC 900:
In our efforts to discover host antiviral proteins, we collaborate with Christine Goffinet (Project C7) to discover novel cellular antiviral mechanisms against human pathogens. During the course of this funding period we started a new project in collaboration with Thomas Krey (Project B10) to obtain the crystal structure of a viral chemokine binding protein (vCKBP) bound to chemokines. This collaborative effort will facilitate the identification of the vCKBP domain involved in the interaction with chemokines and in the functional activity. We perform surface plasmon resonance (SPR) using a Biacore X-100 purchased by the CRC 900 to characterize the interaction between viral proteins and cytokines or neurotrophic factors. We also support and collaborate with other groups interested in SPR under the framework of the CRC 900.
Abel is talking about his research at CRC 900
Abel Viejo Borbolla joined the CRC at the start of the second funding period in 2014. In project B9 he and his group focus on the question of how herpes simplex virus can permanently colonise nerve cells.
Publications of the project B9
Contact

Juniorprof. PhD Abel Viejo Borbolla
Institute of Virology
Hannover Medical School
Carl-Neuberg-Str. 1
30625 Hannover
+49 511 532-4382
Viejo-Borbolla.Abel@mh-hannover.de