Establishing persistent Herpes simplex virus infections in ganglia of the peripheral nervous system
A bit of ointment, a few days of patience and herpes seems to be gone. But the infection is not beaten even once the blisters have vanished from the lips or the genital area. Alphaherpesviruses hide within nerve cells and can be reactivated. In 2012, the WHO estimated that approximately half of the world population is infected with Herpes simplex type 1 and more than every tenth human carries Herpes simplex type 2. However, only some of the infected have disease symptoms (http://www.who.int/mediacentre/factsheets/fs400/en/).
Beate Sodeik and her team investigate how Herpes simplex viruses enter different human cell types and how they get their genome expressed in the host cell’s nucleus. The scientists study proteins of the host cell that help entering herpesviruses to bring their capsids to the nucleus, where they release the viral genome via the nuclear pore into the nucleus. Within the nucleus, the viral genomes are read to induce viral protein synthesis. Subsequently, progeny viral particles are assembled and released. Currently the focus of their work is the infection of nerve cells of the peripheral nervous system, in which Herpes simplex viruses persist for life. The scientists aim to understand the regulation of these factors in the different human cell types to develop novel therapeutic approaches.
Scientific approach
In this project, an experimental model for analysing the cell entry of HSV1 into neurons has been established. The team of Prof. Dr. Beate Sodeik wants to characterise the intracellular signalling pathways activated by HSV1 as well as the specific virus-host interactions. To this end, the researchers use microfluidic chambers, a newly developed polarised cell culture device, which allows for selective infection of the nerve endings – mimicking the infection of free nerve endings in the human skin. For this, they cultivate primary neurons from murine dorsal root ganglia and selectively inoculate either the somata or the presynaptic plasma membrane of axonal endings with virus. This system is suitable for studying HSV1 as well as HSV2 infections.
For analysing cell entry, the researchers generated recombinant, fluorescently labelled HSV1 strains expressing one or two fluorescent protein domains (mCherry, GFP) coupled to viral capsid, tegument or envelope proteins. This enables them to study the intracellular motility of these different viral structures and their interaction with subcellular structures in living cells. Furthermore, they can address whether interfering with specific host factors has any impact on viral fusion with the plasma membrane, endocytic HSV1 entry, axonal transport or targeting of HSV1 capsids to the nuclear pores.
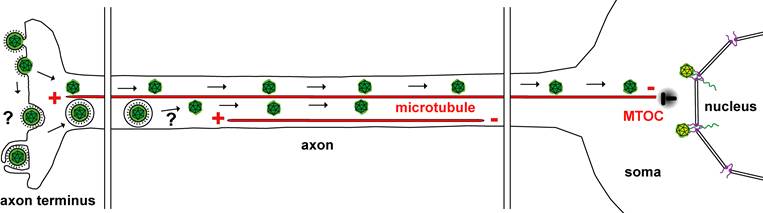
The research group characterises the cellular mechanisms conveying incoming genome-containing HSV1 capsids from the neuronal endings to the soma and the neuronal nucleus. They study the potential function of the microtubule-associated proteins dynein, dynactin and kinesin-1 as well as of specific nuclear import factors and nuclear pore proteins. Many of these host factors occur in different isoforms and form protein complexes, whose subunits are expressed at varying levels in different cell types. The researchers block these proteins by chemical compounds or RNA interference mediated by shRNA expressing lentiviruses. Subsequently, they infect these cells and analyse how interfering with a specific host factor affects the entry of HSV1 into neurons.
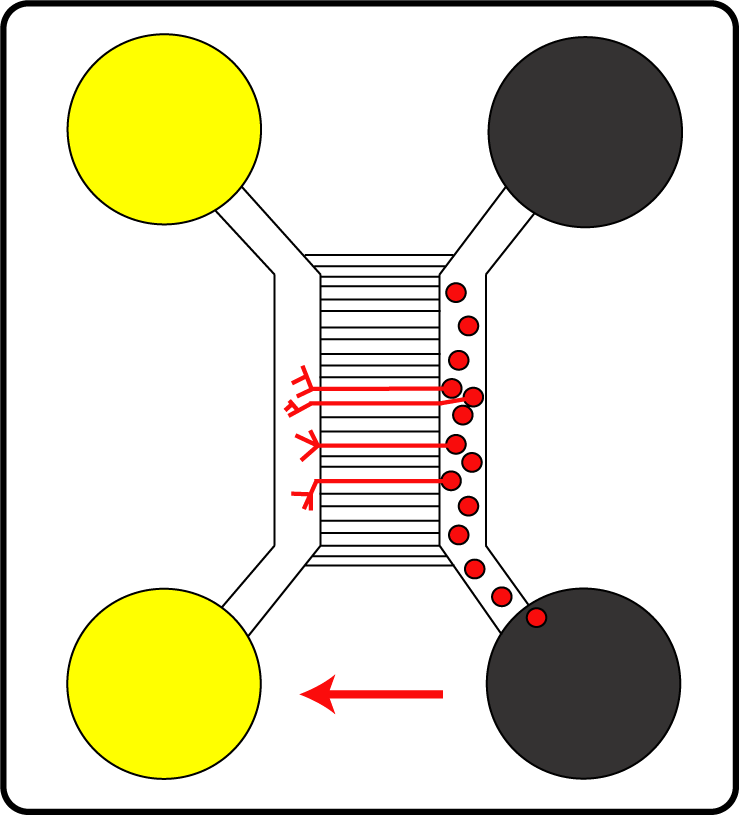
Primary nerve cells (red circles) in special microfluidic chambers: The cell bodies grow and build axons (red lines).
Live-cell imaging of Herpes simplex viruses entering primary nerve cells, cultivated within microfluidic chambers.
Alphaherpesviruses (green) enter nerve cells at the axon endings via fusion of the viral membrane with the axonal plasma membrane. Herpes simplex viruses within endosomes or the icosahedral viral capsids (green) are transported along microtubules from their plus-ends to the minus-Ends at the microtubule organizing center (MTOC) in the cell body of the nerve cell. Incoming capsids dock at the nuclear pores (violet) of the nuclear membrane (nucleus). The viral genomes (green) are released into the nucleoplasm.

Electron microscopy image of an HSV-1 capsid (red) docking to the nuclear pore (yellow) and injecting its viral genome (green) into the nucleus, which is surrounded by the two nuclear membranes (blue).
Beate Sodeik is talking about her research at CRC 900
Beate Sodeik has been part of the CRC since its founding in 2010 and with her team researches how herpes simplex viruses enter different human cells and deliver their genomes into the nucleus of the host cells for expression. The research group mainly focuses on infections in neuronal cells.
Publications of the project C2
Contact

Prof. Beate Sodeik
Institute of Virology
Hannover Medical School
Carl-Neuberg-Str. 1
30625 Hannover
+49 511 532-2846
Sodeik.Beate@mh-hannover.de