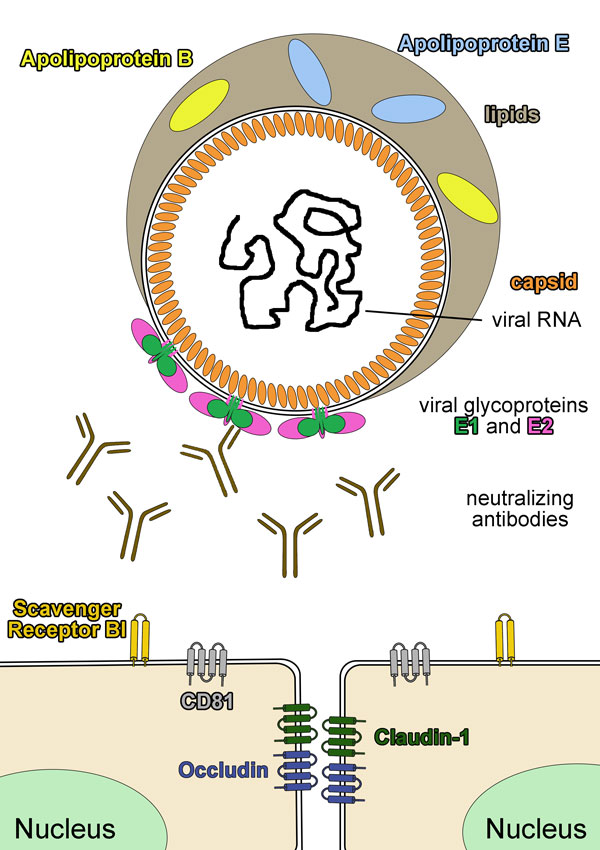
Structural flexibility in the Hepatitis C virus glycoprotein complex as viral strategy to evade the humoral immune system
An estimated 180 million people are infected with hepatitis C virus (HCV) worldwide and ~350.000 HCV-associated deaths per year indicate that HCV is one of the most important viral pathogens in humans. The HCV infection proceeds in about 70-80% of the cases to the chronic phase, mostly because the human organism does not find suitable defense mechanisms to clear the virus during the acute phase. One of the most important defense mechanism of the immune system to counteract viral infections are neutralizing antibodies, which bind to specific surface features of the virus and thereby block the infection of the host cell. HCV has developed numerous strategies to undermine the production and/or neutralizing activity of these antibodies.
Thomas Krey and his group investigate the interactions of neutralizing antibodies with HCV surface features located in the viral glycoproteins – also termed neutralization epitopes – by X-ray crystallography. They determine the 3D structure of these epitopes in complex with specific antibodies. Combined with a functional analysis of the neutralization activity of these antibodies these structures enable the researchers to draw conclusions on the efficiency of these epitopes to elicit particularly potent neutralizing antibodies and therefore on the suitability of these epitopes for informed vaccine design.
To date, our understanding of the three-dimensional arrangement of the viral glycoproteins E1 and E2 present at the virus surface is limited, but such structural knowledge has paved the way for more efficient vaccine candidates in other viral infections like Influenza virus or HIV. Therefore, Thomas Krey and his group concentrate on the structural analysis of an HCV glycoprotein in its native conformation that is assembled in the same way as the one in infectious viral particles.
Scientific work programme
The majority of hepatitis C virus (HCV) infected patients proceed into the chronic phase. To date it remains poorly understood, why the host immune system fails to control the virus during the acute phase. Recent evidence has highlighted the role of neutralizing antibodies (nAbs) in virus clearance and structural studies of nAb fragments in complex with synthetic epitope peptides or a soluble E2 core fragment have provided a significant leap forward for informed vaccine design. The major targets for nAbs are the two glycoproteins E1 and E2 that are incorporated into virions as noncovalent E1E2 heterodimers or as covalently linked multimer. However, to date structural information on HCV glycoproteins are derived from protein fragments or oligopeptides. The principal objective of this project is therefore to determine the 3D structure of the HCV glycoproteins in its native state to understand the spatial arrangement of neutralizing determinants at the virus surface. We will use an integrated structural biology approach including several biophysical and structural biology methods to achieve this goal. In collaboration with Prof. Thomas Pietschmann (project A6) we analyse the effect of these native glycoproteins on the infection of the host cell.
Recently, we and others have shown that a number of epitopes in the HCV glycoproteins that are recognized by potent neutralizing antibodies are conformationally flexible and this flexibility was postulated to constitute a mechanism to evade from nAbs thereby favoring the progress to chronic infection.
In this context, we also aim to characterize the antibody response to these conformationally flexible epitopes found in HCV infected patients. Our results will provide a better understanding of the interactions of the HCV glycoproteins with the humoral immune system that should aid future development of a safe and efficient HCV vaccine.
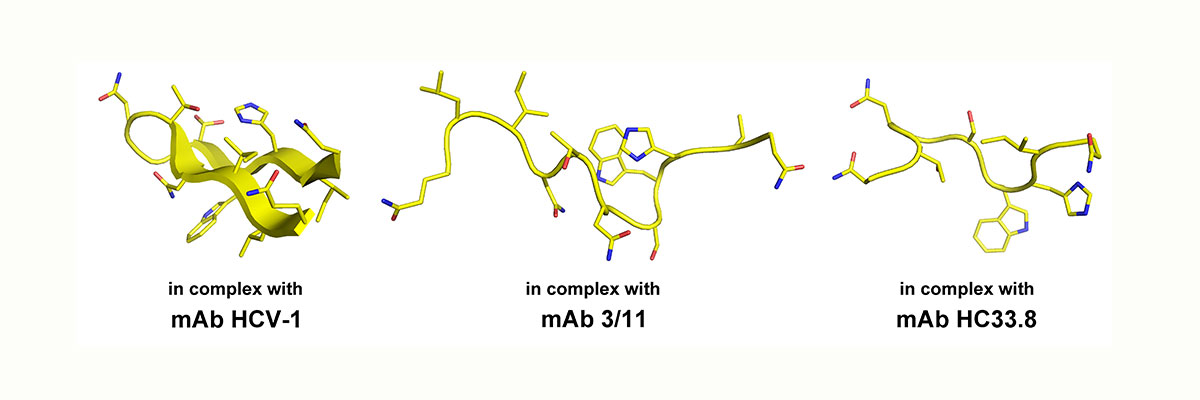
Structural flexibility of an important neutralization epitope within HCV E2. Shown is the conformation of the peptide aa412-423 in complex with three different neutralizing antibodies.
Thomas Krey is talking about his research at CRC 900
Thomas Krey joined the CRC in May 2016. His project B10 addresses the question of how surface structures of hepatitis C virus interact with neutralising antibodies. His research group mainly uses structural biology methods such as X-ray crystallography.
Publications of the project B10
Contact

Prof. Thomas Krey
Institute of Virology
Hannover Medical School
Carl-Neuberg-Str. 1
30625 Hannover
+49 511 532-4308
Krey.Thomas@mh-hannover.de